- Which particle has a negative charge and orbits the nucleus of an atom?
- Carbon plays a central role in many biological reactions because
-
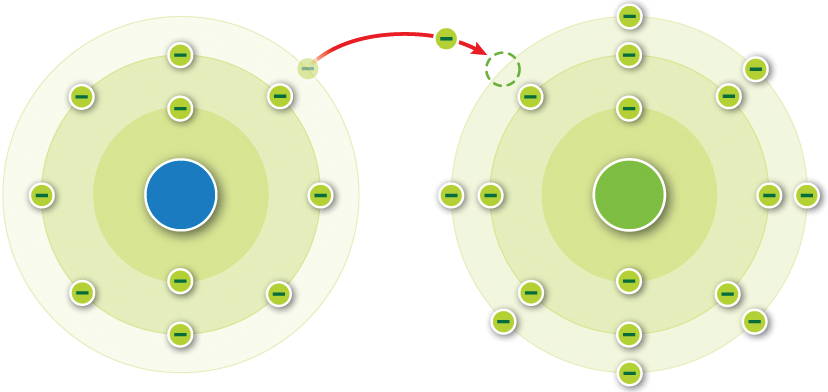 What is the atomic number of the negatively charged ion formed shown in the diagram?
What is the atomic number of the negatively charged ion formed shown in the diagram?
-
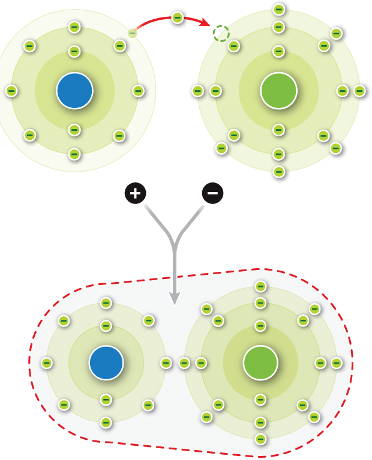 Sodium chloride (NaCl) is composed of alternating sodium and chloride ions held together by ________ bonds.
Sodium chloride (NaCl) is composed of alternating sodium and chloride ions held together by ________ bonds.
- A covalent bond is the sharing of ________ electron(s).
-
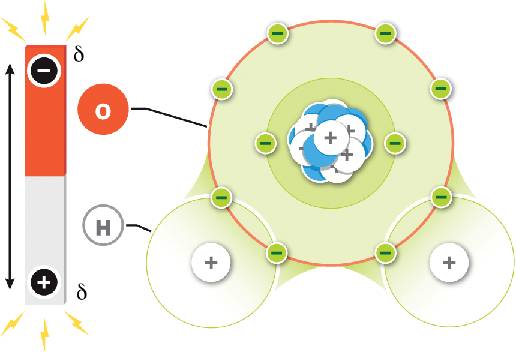 A water molecule has
A water molecule has
-
 The dotted lines in this diagram are ________ bonds due to attraction of partially charged regions between water molecules.
The dotted lines in this diagram are ________ bonds due to attraction of partially charged regions between water molecules.
- Water possesses the following properties EXCEPT:
-
 The property of water that is responsible for surface tension is called
The property of water that is responsible for surface tension is called
- The concentration of hydrogen ions in a solution is a measure of its
- Which kind of macromolecule is made of C, H, and O (carbon and water)?
- The largest sugar from the following list is
-
 Glucose is a
Glucose is a
- Some excess glucose is converted into ________ for short-term energy storage in muscles and liver of animals.
- A protein that is heated can lose its 3-dimensional shape in a process called
- Factors that affect the activity of an enzyme include
-
 The sugar found in DNA (Deoxyribonucleic Acid) is
The sugar found in DNA (Deoxyribonucleic Acid) is